Bard PowerPort Lawsuit
Hundreds of adverse events linked to Bard PowerPort catheters have been reported to the FDA, and lawsuits are being filed nationwide. If you were injured after being implanted with a Bard PowerPort device, you may be entitled to compensation.
Contact Wright McCall at 866-488-1085 today for a free case evaluation.
Bard PowerPort Lawsuit
Bard PowerPort Device Failures Linked to Serious Health Risks
If you or someone you know suffered harm from a Bard PowerPort implant, Wright McCall’s experienced product liability attorneys can help. To speak with an attorney, call 866-488-1085 or submit a free case evaluation.
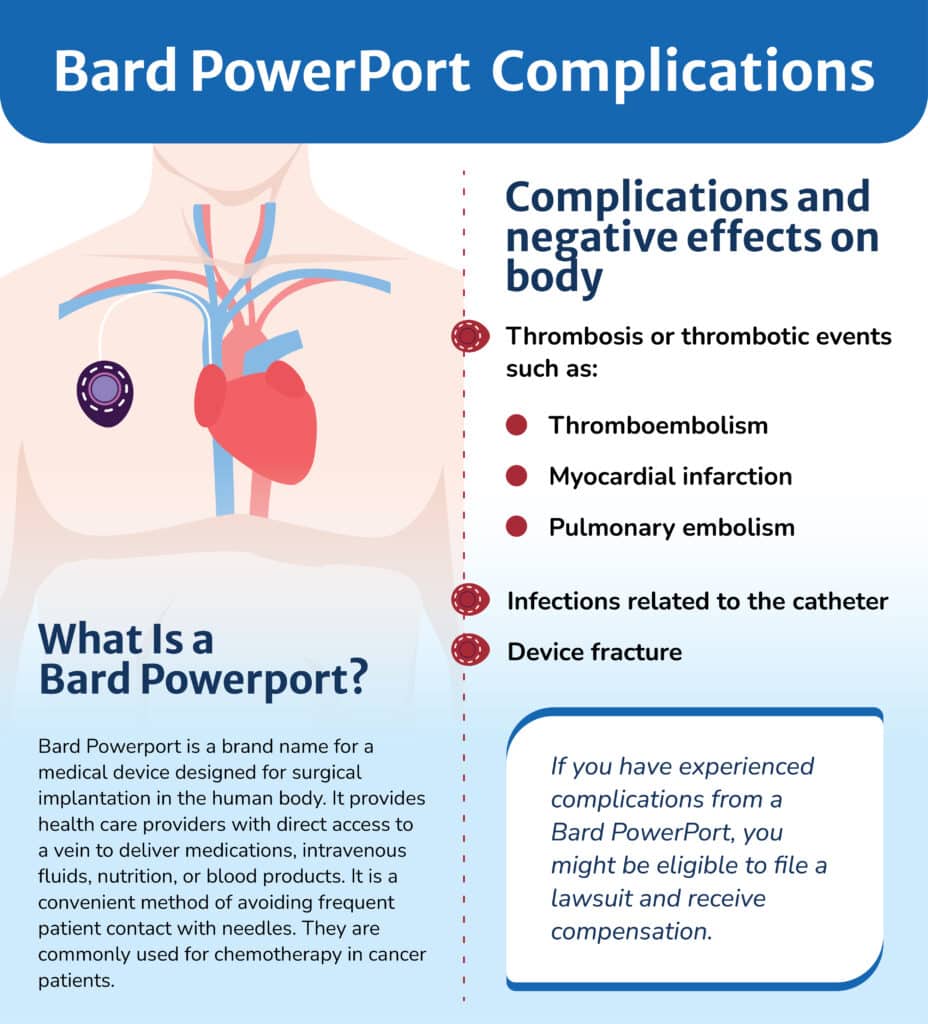
About the Bard PowerPort
Implantable ports are often used for patients requiring frequent or long-term IV treatments, blood draws, or transfusions—such as cancer patients undergoing chemotherapy. These devices allow direct access to the bloodstream.
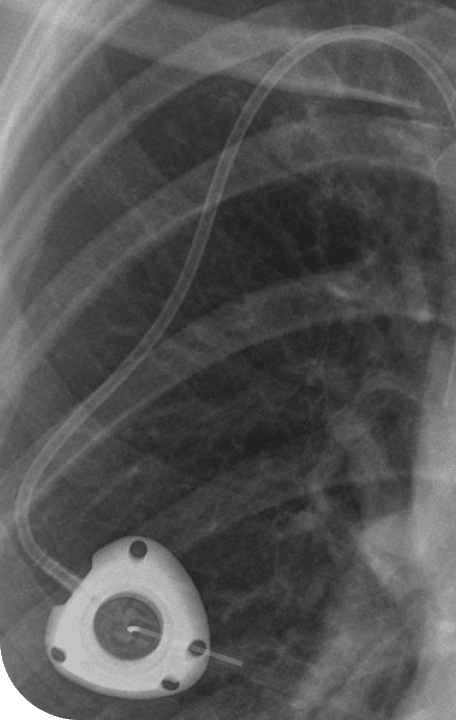
The PowerPort ClearVUE Implantable Port, marketed by Bard, is designed to be flexible and resistant to surface degradation and stress cracking. It is constructed from polyurethane plastic with a septum (needle access point) and a catheter that leads into a blood vessel.
Injuries Linked to Bard PowerPorts
Unfortunately, Bard PowerPorts have been associated with higher-than-expected injury rates. Some of these injuries occur when metal needles rub against the inner plastic catheter, causing small holes or scratches. These micro-damages may allow bacteria to accumulate, potentially leading to severe or even fatal infections and sepsis.
Further, the device includes barium sulfate mixed into the plastic to make it visible on imaging. Once implanted, that additive can weaken structural integrity, increasing risks of degradation, fracture, or migration within the body. Some users have also experienced blood clots.
Recent Lawsuit Developments (as of August 2023)
The number of Bard PowerPort lawsuits has grown significantly, with dozens of cases filed across multiple federal jurisdictions. To streamline handling, the U.S. Judicial Panel on Multidistrict Litigation (JPML) consolidated these cases in the U.S. District Court for the District of Arizona, under Judge David G. Campbell. He now oversees the common legal issues and bellwether trial selections.
Could You Have a Case?
You might qualify for a claim if, following implantation of a Bard PowerPort, you experienced one or more of the following:
-
Port device breakage or fracture
-
Infection diagnosed more than 90 days after implantation
-
Blood clots
-
Device migration
-
Cardiac arrhythmia
-
Tissue, vessel, or organ perforation
-
Necrosis around the port site
-
Cardiac or pericardial tamponade
How to Move Forward
If you had a Bard PowerPort implanted and suffered injury, you should contact Wright McCall for a 100% free case review. Our attorneys accept cases from across the United States.
Call 866-488-1085 now or fill out our contact form to share your story.
Complications Linked to Bard PowerPort Devices
Lawsuits claim that Bard PowerPort catheters may shed particles that enter the bloodstream, potentially traveling to internal organs. This can cause serious organ damage and life-threatening infections.
Reported Risks and Injuries
Patients with Bard PowerPort implants have been reported to face an increased risk of:
-
Thrombosis or thrombotic events, including:
-
Thromboembolism – a dangerous blood clot that blocks blood flow in the veins
-
Myocardial infarction symptoms – heart attack-like symptoms such as chest pain, dizziness, or difficulty breathing
-
Pulmonary embolism – a clot that travels to the lungs, cutting off blood flow and requiring emergency treatment
-
-
Infections linked to the catheter
-
Device fracture or breakage
Additional Complications Reported
Some patients have also experienced:
-
Severe or persistent pain
-
Tearing of internal tissues or structures
-
Hematomas (bleeding under the skin)
-
Cardiac arrhythmia (irregular heartbeat)
-
Fluid buildup around the heart (pericardial effusion)
-
The need for corrective or emergency surgery
Device Breakdown Concerns
According to complaints, barium sulfate particles within the PowerPort may degrade over time. As the material breaks down, it can leave pits, cracks, and microfractures that weaken the device. This increases the risk of:
-
Device malfunction
-
Breakage or fracture
-
Migration to other parts of the body
Contact Wright McCall
“A groundbreaking new study shows that some popular tampons may have toxic heavy metals in them, such as arsenic and lead. Studies show that, over time, these toxins can cause severe damage to a human. NBC News’ Dr. Natalie Azar has more information.”
– NBC News
Bard PowerPort Case Timeline
What Is a Bard Powerport?
Liability for Defective PowerPort Catheter Defects
Manufacturers of medical devices are required to ensure that the products they make available to the public are safe and effective.
The complaint allege that when C.R. Bard began receiving adverse event reports, it may have prevented hundreds of injuries and deaths by doing even one of the following:
- Recall the product
- Remove the barium sulfate from the product
- Encapsulate the barium sulfate
- Use a different polymer formulation
- Update the warning label to disclose the known risks
Instead, Bard continues to market the product as safe and effective.
Complaints allege that not only did the company fail to take steps to protect the public, but it may have deliberately concealed the product’s harmful effects from the public.
Contact Wright McCall to Start Your Claim Today
If you or a loved one received a Bard PowerPort implant and later suffered complications such as catheter migration, fracture, infection, organ damage, or life-threatening blood clots, you may be eligible to take legal action against Bard and Becton Dickinson.
Bard PowerPort Devices Involved in Lawsuits
-
Bard Power-Injectable Implantable Ports (PowerPorts®)
-
BardPort®, SlimPort®, and X-Port® Implanted Ports
-
Power-Injectable Implantable Ports with Chronoflex Polyurethane Catheters
-
PowerPort Implantable Port
-
PowerPort ClearVUE Slim Implantable Port
-
PowerPort Duo MRI Implanted Port with 9.5 Fr. Dual Lumen Chronoflex Polyurethane Catheter
-
PowerPort Implanted Port with Groshong Catheter
-
MRI PowerPort Implanted Port with 9.6 Fr. Silicone Catheter
-
Titanium PowerPort ISP Implanted Port with 6 Fr. Chronoflex Polyurethane Catheter
-
Titanium PowerPort ISP Implanted Port
About Wright McCall Attorneys
Wright McCall is a nationally recognized personal injury and product liability law firm. We handle a wide range of claims involving dangerous medical devices, pharmaceuticals, and consumer products. Our team is committed to advocating for clients harmed by defective products and unsafe practices.
Contact Us — Available 24/7
We make it easy to reach an attorney from anywhere in the U.S. Call 866-488-1085 or submit a request for a free consultation.
